Net zero, part 3: production
Point source capture and cleantech works for most things. Ammonia isn't one of them.
This is part of a series of posts on how to pause climate change using technology and policy:
Net zero, part 3: production (you are here)
Net zero, part 5: stopping climate change
In the short term, it’s better to pause climate change than revert to a pre-industrial climate. To pause climate change, it’s sufficient to achieve net zero emissions across the economy. Here we’re going to focus on getting to net zero in production of products like plastics, steel, concrete, and ammonia.
Like with transport, carbon-conscious manufacturing will usually be more expensive than it is today. I’m going to assume that there’s a $100/ton carbon tax and ask how production costs will change in response. Increases of over 10% are considered concerning.
The focus is on direct CO2 emissions from each product, not emissions from energy sources (addressed in the first post) or other forcing contributions. I’ll focus on products that are fundamental to our society and/or produce a large amount of our CO2 emissions.
Petroleum refining
Petroleum refining is the first step for crude oil after it has been pumped out of the ground. This is how we get gasoline and the precursors for chemical products.
There are two main energy inputs to the process, heat and hydrogen. We covered industrial heat in the first part of this series. The roughly 400°C temperature requirement is met today by burning fuel. The easiest way to make this green is point source capture, but thermal storage combined with renewables will soon beat this on cost.
Only 37% of the emissions from refining come from the process itself, most of the rest comes from producing heat. This paper finds that refining produces 40 kg of CO2 per barrel, including heating. The DAC cost comes out to $1.48 per barrel, roughly 2% of the cost of a $70 barrel of oil. Using point source capture is feasible on refineries and could lower capture costs by over 50%.
Note that with the carbon tax from the transport section, the net effect on gas prices would be a 6-7% increase in per-mile driving costs. Switching to a hybrid would halve that.
Plastic
Plastic production has few CO2 emissions per unit of product. The carbon is the product, so you don’t want to burn it. Indeed, the reactions to produce most plastics do not produce CO2 on their own. Rather, the emissions from the precursors and the heat required for the reactions are the source of emissions.
Plastic also does not degrade for hundreds of years, particularly if stored properly in a landfill. The net effect is that plastic adds very little CO2 to the atmosphere relative to other products.
So the “direct” CO2 emissions from plastic are very small. As a proxy, I’ll look at how much a carbon tax will increase the price of ethylene. Ethylene is the precursor to polyethylene and PVC, which represent slightly less than half the global plastics market by mass.
Like with plastics, steam cracking to produce ethylene has little direct CO2 emissions. Rather, most of the emissions come from using fossil fuels for heat. This paper claims that over 90% of the emissions come from the furnaces. This page estimates 1.2 tons of CO2 per ton ethylene. The 0.12 tons of direct emissions cost $12 with the carbon tax, 2% or less of the $600-$800/ton price tag.
The price impact on plastics is even smaller as ethylene feedstock is only a portion of plastic manufacturing costs. The plastics industry can probably just eat the price of carbon taxes. Point source capture, heat from renewables, and recycling via pyrolysis can lower the tax impact further.
Cement
Cement production is interesting because it produces CO2 from heating limestone rather than burning fuel1. 60% of cement productions emissions come from heating the limestone to produce lime. The rest come from industrial heat.
Let’s see what the price would be with DAC. Each ton of cement produces 0.9 tons of CO2, 0.54 t of which are direct emissions. Over concrete’s lifetime, it reabsorbs roughly 30% of the CO2 it released, bringing us to $38 of DAC per ton of cement. Cement is usually around $100/ton, so the DAC would add almost over 40% to the price of cement.
Fortunately, CO2 comes out of limestone in a concentrated form, so we can capture it on site or try to reincorporate it into the cement. Leilac captures cement emissions and may reach $40/ton of CO2 capture. CarbonCure may recycle 60% of the CO2 from cement. Alone, each would roughly halve the carbon tax, but if we stack both together, capturing the unused CO2 costs less than $9 per ton of cement, a reasonable green premium.
There are many more ideas for reducing the emissions of concrete, discussed in the appendix.
Steel
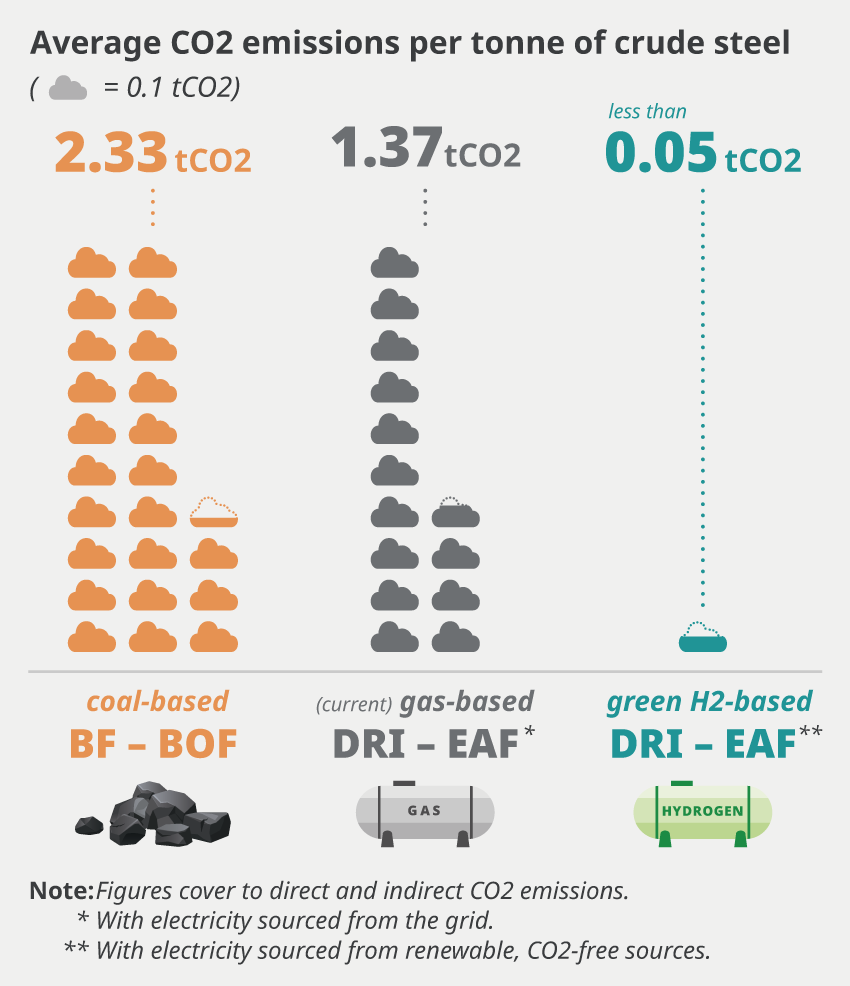
Making new steel from iron ore requires using metallurgical coke (carbon) to remove oxygen from the ore, generating CO2. Steel produces about 2.1 tonnes of direct CO2 per tonne of steel2. That costs $210 with DAC. It’s hard to find a consistent steel price, particularly because there are so many types, but $800/ton seems reasonable. So DAC would make it 26% more expensive. We need to look elsewhere.
One option is to reduce the steel with hydrogen from natural gas using steam methane reforming (SMR). Apparently 7% of steel produced today starts with this form of direct reduced iron. This site claims emissions could fall to 1.37 tonnes with natural gas direct reduced iron. Combine with $50/ton point source capture, and the green premium is only 9%.
What’s interesting is that hydrogen from natural gas can be cheaper than metallurgical coke. A ton of steel requires 780 kg of coke. At $140/ton that costs $109 per ton of steel. You only need 50 kg of hydrogen to produce a ton of steel, so as long as it’s $2/kg or less it should be cheaper than using coke. Assuming SMR provides hydrogen at $1.25/kg, DAC at $100 per ton brings this to $1.80/kg, which is already competitive. Point source capture makes the numbers look even better3.
Steelmaking seems poised to switch to greener alternatives. While hydrogen plus carbon capture looks cheaper on paper, in practice there will be switching costs. Slowly phasing in a carbon tax can get the industry to switch over to greener production methods with a small price impact.
Aluminum
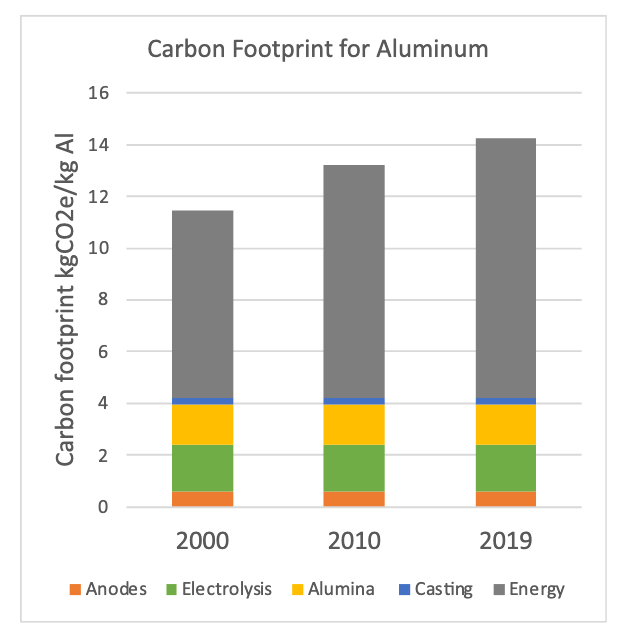
Aluminum is called solid electricity for a reason, the electrolysis process requires a lot of thermal and electrical energy to work. Fortunately, most of the energy input can be replaced with green sources. The real challenge is that aluminum production uses carbon anodes which slowly convert to CO and CO2 over time.
There are efforts to make carbon free electrodes for aluminum electrolysis, but let’s assume that doesn’t pan out. The carbon anode contributes about 1.5 tons of CO2 per ton of aluminum adding $150 of DAC to the $2700 price tag. At less than a 6% increase, I think the aluminum industry will be fine once it switches to green sources of thermal and electrical energy.
In response to the tax, the recycling rate may increase somewhat. This paper suggests that if on-site renewables get cheap enough, aluminum may replace steel as our main structural metal.
Ammonia
Here’s the thing with ammonia:
It feeds the world, making crops roughly 4x more productive. Half the nitrogen in your body came from ammonia production. Making it more expensive means less food which is bad.
Due to several revolutions in chemical engineering4, ammonia is quite cheap. That means it’s sensitive to small changes in input costs. So a carbon tax is going to make it significantly more expensive which we established is bad.
Ammonia needs to be combined with fossil carbon to make it more effective. More than half of ammonia is combined with CO2 from SMR to produce urea (which is easier to ship). Urea can be further modified with fossil carbon to make controlled release fertilizers. The side effect is that this fossil carbon ends up in the biosphere.
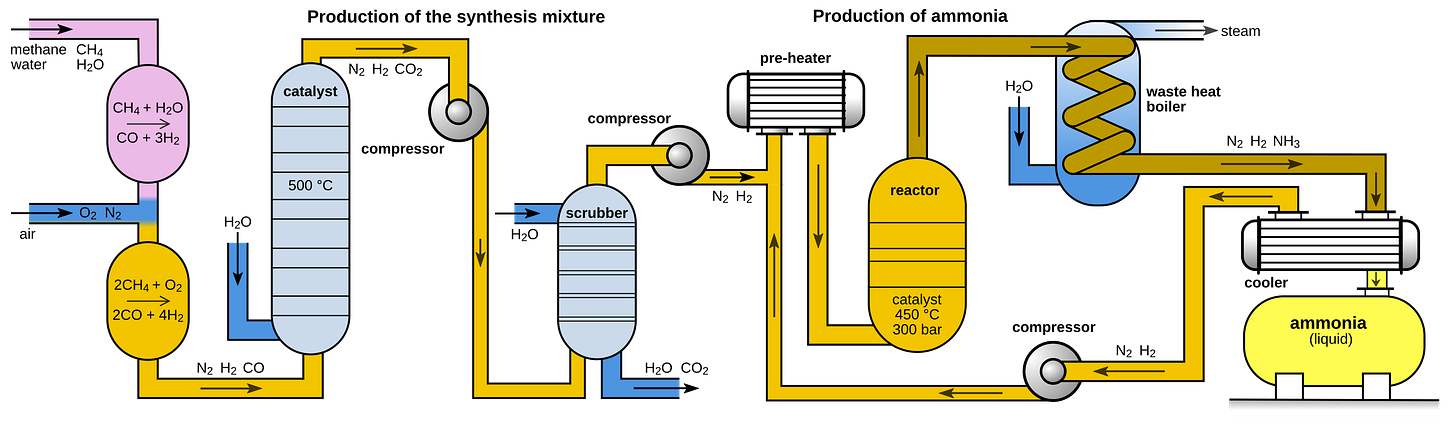
Let’s see how much point source would add to the price of fertilizer. 1 ton of ammonia needs 0.9 tons of natural gas which produces 2.48 tons CO25.
Ammonia costs about $400/ton in the US so DAC at $100/ton means a 62% increase in price. Even with cheap point source capture at $20/ton that’s still a 12% bump. And if you’re making urea, much of the carbon cannot be captured at the source because it’s going to be applied to crops.
Given all of this, I’m tempted to say we should just leave ammonia alone for now and attack the issue from a different angle. Ammonia production constitutes 1-2% of global CO2 emissions, so we can afford to wait, say, 20 years for a better solution. The appendix has some other notes on green ammonia.
Clean hydrogen sources
Eventually green hydrogen might get cheap enough to replace hydrogen generated by SMR. In the appendix, I talk about why that’s challenging and the requirements for competitive green hydrogen.
One more speculative option is to use white hydrogen for ammonia production. This is a recently discovered form of geological hydrogen buried deep under ground. If you site a Haber-Bosch plant next to a source of white hydrogen, you could potentially achieve lower costs than SMR. At this point it is unclear how common these resources are or how economically they can be extracted. The fact that we are only just discovering white hydrogen is a sign that it it either rare or hard to extract. But perhaps improvements in drilling technology will change that.
Regardless of the source, producing urea is probably still not viable. That’s because to make green urea you need to capture and use carbon from the air. That’s likely more expensive than the $100/ton I’m assuming for enhanced weathering because you need to get the CO2 off of your absorbent for use. At that price, urea production becomes uneconomical.
Alternatives to urea
Because urea emits carbon and requires carbon, the industry could shift to nitrates as a nitrogen source. Nitrates today are made from ammonia using the Ostwald process.
It always struck me as wasteful to do all that work making hydrogen and ammonia just to burn the hydrogen and make nitrates. There’s an obsolete process for making nitrates from air directly called the Birkeland-Eyde process. Could on-site renewable energy make this viable again? See point 3 here for more.
The nitrate ion needs a counterion. Nitric acid has hydrogen as the counterion, but is too acidic for plants and dangerous to work with for many reasons. Ammonium nitrate is a common fertilizer and gets close to the percent nitrogen found in urea. Potassium nitrate has a much lower percent nitrogen because of the weight of the potassium ion, but potassium is already an important component of fertilizers, increasing the value. It’s also possible to make potassium nitrate without any hydrogen.
In any case, nitrates have lower percent nitrogen than urea so shipping costs will be higher. Nitrates also have the drawback that they can be used to make explosives which is a proliferation risk. It would be interesting to see a comparison of fertilizers in terms of handling difficulties, nitrogen use efficiency, and release of NOx from the soil. On a first pass, ammonium nitrate seems more cost effective and less emitting than urea, though shipping costs are higher.
Ammonia demand is set to fall
One odd fact about ammonia is that demand is flat or falling in most places. The world is approaching 'peak fertilizer' due to more efficient use of fertilizer and fertilizers that are more effective per unit of nitrogen. These are important factors to consider when switching away from urea. Less efficient fertilizers could raise demand and net emissions.
Falling demand isn’t entirely good news. Sub-Saharan Africa uses very little fertilizer due to a lack of domestic production and high shipping costs in a continent that is essentially landlocked. This is part of the reason why agricultural productivity is so low there.
In addition, virtually all world population growth for the rest of the century will be in Sub-Saharan Africa. The confluence of these factors suggest a different strategy for fertilizer production. Perhaps local generation of nitrate fertilizers can simultaneously lower fertilizer costs in Africa while avoiding the emissions that would normally entail.
Further reducing ammonia demand
I think we can lower fertilizer demand even further by:
Using fertilizers with higher nitrogen use efficiency.
Higher precision timing and delivery of fertilizer.
Making nitric acid on site with nitricity.
Recycling nitrogen from farm runoff.
Using urease inhibitors and nitrification inhibitors to prevent bacteria from breaking down fertilizer.
Adding nitrogen-fixing microbes to the soil with Pivot Bio and Kula.
GMO’s that are more productive per unit of fertilizer.
Conclusion
Products like gasoline, plastic, steel and aluminum can survive a carbon tax with only small (or negative) increases in price. Cement requires a combination of point source capture with other tricks to hold the green premium below 10%. For these products, I expect the green premium will add little to the cost of final goods. In the future, these processes may lower costs further by moving to locations with cheap renewables and opportunities for point source capture.
Ammonia, on the other hand, is beyond help. We need to move away from making urea and build cheap sources of clean hydrogen. Given its importance, it may be wise to have a slower pace of emissions restrictions on fertilizer.
While this list isn’t exhaustive, most other materials are made at a much smaller scale with fewer CO2 emissions per unit of product. Most can decarbonize in a similar manner by using point source capture and renewable energy to eliminate emissions.
Appendix
Cement solutions
The (good) solutions I’ve seen can broadly be categorized as point source CO2 capture, incorporating CO2 into concrete, recycling concrete (into cement or aggregates), making concrete stronger, or switching to a different material.
Articles and papers
Good review of decarbonizing cement by Hannah Ritchie
What Will It Take to Decarbonize Cement? | Breakthrough Energy
Will Stone Replace Steel and Concrete?
So you want to use less concrete
Electric recycling of Portland cement at scale | Nature
Decarbonization of cement production by electrification. “With an electricity price of 50 €/MWh, the partially electrified alternatives (OC-HK, eC-afK) showed competitive additional cost of clinker (87 €/tclk) and cost of avoided CO2 (101 €/tCO2) against a benchmark case, though higher than the cost of the best CO2 capture technologies from the literature.” I think on-site renewables can be 2-3x cheaper than this.
Companies
Coolbrook Rotodynamic Heater
SaltX Technology Electric Arc Calciner
Reversa® Binder Concrete Technology | CarbonBuilt
Fortera ReCarb® Plant in Redding, CA, USA
Don’t use biomass
Plants and animals aren’t perfectly efficient at converting energy into food, so there will always be some available biomass that can be used to make organic products. Add in sewage, compost, and mechanical brush removal and this could be a cheap source of fuel right?
There’s a reason we don’t use biomass for chemical products without government subsidies. Biomass is less useful, less energetic, and harder to work with than fossil fuels. To upgrade it, you need green hydrogen, renewables, new reactors, and new supply chains. It also emits little net CO2, so using it isn’t critical. Fuel and DAC is better.
However, in the next post, I’ll talk about the benefits of using biomass on site for food production.
Don’t reuse point source CO2
I’ve come to the conclusion that utilizing point source CO2 for other products doesn’t make sense. If you’re getting the CO2 from a power plant, you’re going to need a lot of energy just to reduce the CO2 to a usable form (like methanol). It’s better to make the product from fuel directly rather than burn the carbon and then unburn it.
Point source CO2 from concrete avoids this issue. But producing products from this carbon is silly for the same reason that e-fuels are silly. Converting point source CO2 to fuels using renewables and green hydrogen and DAC for the eventual emissions is necessarily more expensive than just doing point source capture.
The only benefit I can see for utilizing point source CO2 is splitting the carbon tax between products. For example, if an ammonia plant used the CO2 from a concrete plant, they could split the carbon tax. This no longer makes sense if point source capture is less than half the cost of DAC. Also, siting industrial processes next to each other is hard.
What green hydrogen needs
For now I think it’s better to use SMR and point source capture for hydrogen. Green hydrogen has not yet scaled like other technologies. You would need:
Less than $10/MWh electricity (only achievable with solar and no batteries).
Greater than 0.3 utilization of the electrolyzer. Only achievable in places with lots of sun and many hours of sunshine. Being near the equator limits sunlight hour change between seasons which should keep costs down.
Less than 80 kWh/kg hydrogen (achieved today by alkaline electrolyzers).
Less than $100/kw electrolyzers. Today’s alkaline electrolyzers are around $200/kw, Terraform claims they are under $100/kw with theirs, though it’s not clear if it’s alkaline or PEM.
Greater than 10 year lifetime (achieved by today’s electrolyzers)
Interest rate less than 5%. Would require a government subsidized loan in today’s environment.
Put that all together and you get $1.20/kg of hydrogen. Even on site, that doesn’t obviously beat SMR, though it would work if SMR had to pay a carbon tax. For off site use, you need to transport the hydrogen. The best plan I can think of adds $0.35/kg of hydrogen bringing the total to $1.55 for off-site green hydrogen.
The only factor above I can see getting dramatically better is the lifetime. Today’s electrolyzers can last for 25 years. Getting a cheap one to last that long would bring the on-site hydrogen price down to $1.02/kg.
After that, you need to think about making the electrolyzer even cheaper with a stainless steel anode, recycling the PPS plastic, recycling the electrodes, better/cheaper diaphragms, and higher operating temperatures/pressures.
Eventually the price of natural gas will grow (since it’s an exhaustible resource) and green hydrogen will take the charge. But we shouldn’t wait.
More notes on ammonia
Birkeland-Eyde could work. Though I would think plants would grow more efficienctly with a reduced form of nitrogen. Nitrates are harder to transport and heavier. Nitrates may run off faster and thus require nitrase inhibitors which adds to costs and fossil carbon.
Ammonium nitrate has fewer CO2 emissions than urea. Though it’s more complicated to make because of the Ostwald step. Most controlled release fertilizers are urea-based, so AN might be less efficient. AN comes with proliferation risks, but this may be addressed with better surveillance.
The Frank-Caro process is interesting, but still needs fossil carbon and it’s more expensive than haber bosch. Potentially less capex intensive and thus could work with intermittent energy. Alternatively, could you ship the cyanamide directly? Or does the CaCO3 sequester carbon in the soil? EDIT: after thinking about this more, I’ve ruled out using Frank-Caro to produce ammonia and ruled out using calcium looping to produce cyanamide alone. See next point.
EDIT: But, producing calcium cyanamide is interesting. By importing calcium carbonate, converting to CaCl2 with chlorine and water, making calcium metal via electrolysis, making calcium carbide using coal, turning that into calcium cyanamide (and optionally converting into potassium cyanamide) and using that as fertilizer is carbon-neutral. Why? Because the calcium cyanamide turns into ammonia and calcium carbonate in the soil. At worst, this leaves carbonate in the soil, at best it runs off into the ocean and captures more carbon while buffering the ocean and feeding carbon to marine life. You get carbon neutral (or negative) fertilizer using standard processes and no hydrogen. This plan has the drawback of high complexity and high shipping costs due to the weight of the calcium counterion.
Chemical looping synthesis is orders of magnitude (point 3, “this paper”)more energy intensive than Haber-Bosch. Other metals can get closer but still require heat and pressure, the main thing we wanted to avoid with chemical looping synthesis.
Switching to ammonia solutions. This is used in the US but probably too heavy for international shipping. Ammonia is pretty corrosive as well.
Though fuel is burned for heat, addressed in the energy post.
I’m not including the processing and heating emissions of 0.21 tonnes.
$50 to $20 per ton point source means $1.53 to $1.36 per kg of hydrogen respectively.
Haber-Bosch, steam-methane reforming, LNG transport, ammonia transport, fracking, etc.
There are ways we can cut down the estimate a little. For example, some of the CO2 in urea might be stored long term in the soil. Using fertilizer also means more plants than there otherwise would be and thus more stored carbon. It also reduces agricultural land use. Algal blooms caused by fertilizer runoff are bad for other reasons, but might enhance the ocean’s CO2 sink. That said, I doubt these factors change the net emissions much. On the other side of the ledger, nitrogen fertilizers evolve N2O, which is a greenhouse gas with a 120 year lifetime.


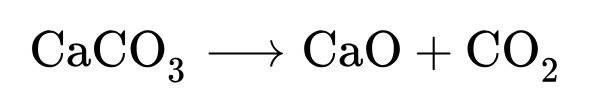
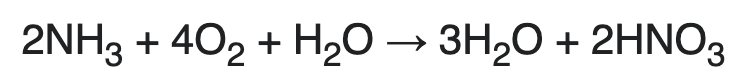
In high income countries, the majority of the cost of construction is land, labour and permitting costs. Steel and cement don't contribute much to the final cost of the building. Plus most building regulations are local. Local progressive jurisdictions can get together and have a mandate for using green steel and cement assuming green premium is less than (let's say) 80%. Final cost of the building will go up by less than 3-5% probably.
As I said in previous comment, some costs matter more than others.
The same thing can be done for single use plastics. Mandate carbon neutral plastic production and even if the price increases by 300%, it probably won't show up in the CPI.
Again I prefer a neutral carbon tax, but these are the cases where the targetted policies make the most sense.
This is all very interesting and good research, but in a competition of nations where you can’t enforce rules, aren’t we doomed to always use the cheapest?